How Many Valence Electrons Are in the Nitrate Ion
C How many valence electrons does one NO ion have. D two double bonds and no.

How To Find The Valence Electrons For No3 Nitrate Ion Youtube
9 How many valence electrons are in the nitrate ion.

. Nitrogen and oxygen are located at VA and VIA groups respectively in the periodic table. E Can you draw equivalent resonance structures for this ion. Electrons in one-third of a π bond 23.
Hence oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. Therefore the valence electrons of nitrogen are five. Electrons in one π bond 2.
Due to -1 charge another electrons is added. We use cookies to give you the best possible experience on our website. So nitrogen has five electrons in its valence shell.
In oxygen atom there are six electrons in its valence shell. Also to know how many valence electrons does c2h4 ethene have. Total valence electrons given by nitrogen atom 5.
There are two oxygen atoms in NO 2 Therefore. Nitrates salts with NO 3- are frequently used in agriculture as a fertilizer. Again the iron atom donates two electrons in 4s orbital and an electron in 3d orbital to convert iron ion Fe 3.
Nitrate ion NO3- can form 1 bond as in NaNO3. The total number of electrons in a valence shell is called a valence electron. Brainly UserBrainly User.
C three bonding and one unshared pair of electrons. View the full answer. A 18 B 22 C 23 D 24 E 26.
In the ion NO3 there is 1 atom of nitrogen and 3 atoms of oxygen. Fe 3e Fe 3. This is in part to their high solubility in water.
The nitride ion N-3 is formed when an atom of nitrogen gains 3 electrons. You have nitrate which is very stable and there is a reason for it. Up to 25 cash back How many valence electrons are in the Lewis structure of a nitrate ion NO2.
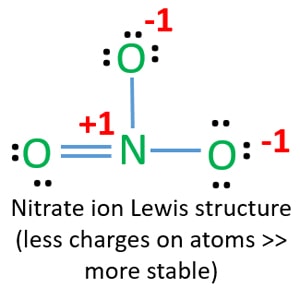
D Draw a valid Lewis structure for the NO3 ion below. Ans In this case the 1 charge carried by the nitrate anion NO3- corresponds to one extra electron. Nitrogen atom has 5 valence electrons ec 25.
The bond order for the N-O bond in the nitrate ion is said to be 43. How many valence electrons are in the nitrate ion. Oxygen has a higher electronegativity than nitrogen and therefore it takes one electron and gets reduced to -1 to have a full filled p orbital 8 valence electrons.
In a non oxidizated state you have 6 valence electrons outer s and p orbitals for oxygen. If so indicate these below. Total valence electrons given by nitrogen atom 5.
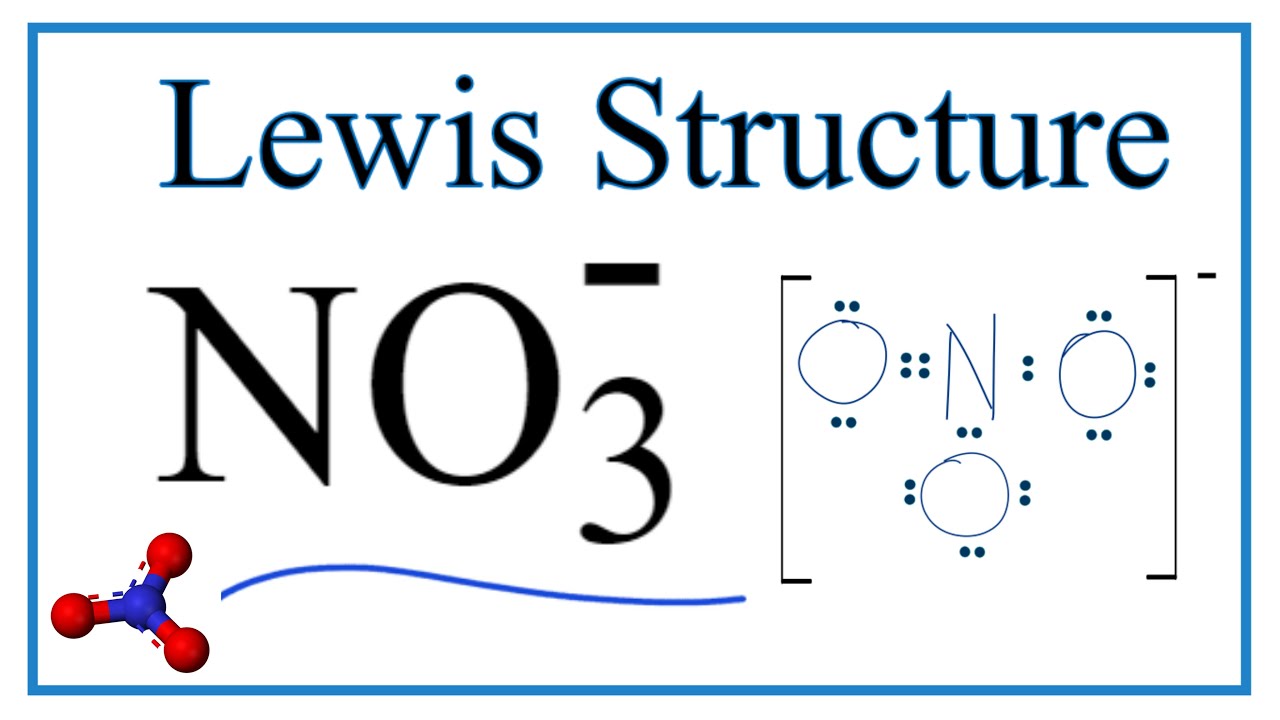
3 How many valence electrons are in the nitrate ion. 24 valence electrons. A step-by-step explanation of how to draw the Nitrate Ion Lewis Dot StructureFor the Nitrate Ion Lewis structure calculate the total number of valence elec.
So nitride ion will have 53 8 valence electrons. Oxygen has 6 valence electrons each and nitrate has a - 1 charge to add another valence electron. It also has one negative charge.
In oxygen atom there are six electrons in its valence shell. Valency is the maximum number of bonds which the element can form and is usually related to the number of valence electrons. But keep in mind that.
Construction of NO3 Lewis Dot Structure. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table. Also there is a -1 charge on the nitrate ion.
The nitrate ion as represented by the hybrid has two π electrons. There are three oxygen atoms in NO 3- Therefore. To determine the number of valence electrons for NO3- the Nitrate ion well use the Periodic Table.
Similarly what is the Lewis structure of nitrate ion. So nitrogen has five electrons in its valence shell. The three bonded atoms sulfur S nitrogen N and C produce an ion with a linear shape.
Drawing the Lewis Structure for NO3-. For this iron ion Fe 2 has a total of fourteen valence electrons. B one bonding and three unshared pairs of electrons.
This electron configuration shows that iron ion Fe 2 has three shells and the last shell has fourteen electrons. B How many valence electrons do three oxygen atoms have. The electron configuration of nitrogen shows that the last shell of nitrogen has five electrons2s 2 2p 3.
A two bonding and two unshared pairs of electrons. How many valence electrons a does ICl4 ion have. Now for a given molecule the number of valence electrons is.
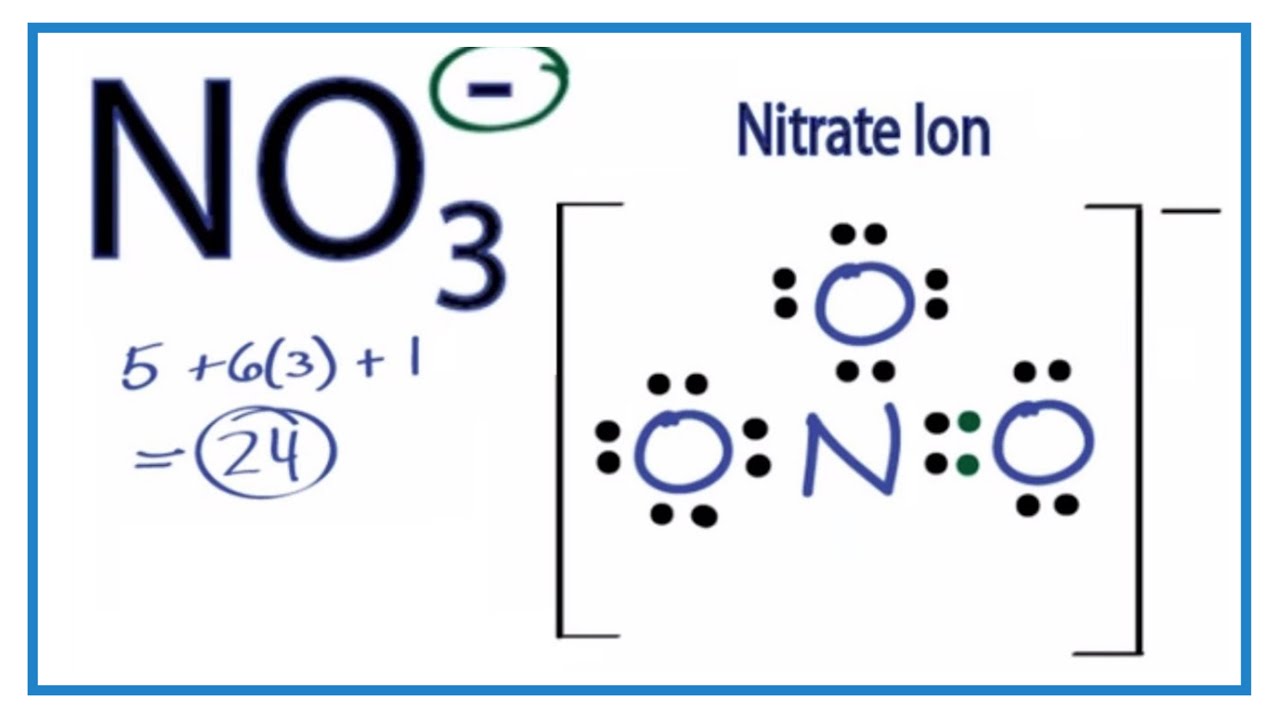
N 5 e- 3 Oxygens 18 e- NO3 has a -1 charge so add another electron for 24 total. Organizing the Periodic Table by Group skipping the. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond.
Technically elements have a valency. N has 5 valence electrons. Total valence electrons given by oxygen atoms 6 2 12.
For C 2 H 4 you have a total of 12 total valence electrons. If you are asking about ICl4- ion then there are 28 valence electrons and 8 binding onestotal of 36 electrons. There are 24 valence electrons available for the Lewis structure for NO 3-.
In your case the nitrate anion contains one nitrogen atom three oxygen atoms A quick look in the periodic table will reveal that nitrogen is located in period 2 group 15 which tells you that it has a total of five valence electrons. The central atom in the chlorate anion ClO3-is surrounded byA two bonding and two unshared pairs of electronsB one bonding and three unshared pairs of electronsC three bonding and one unshared pair of electronsD two double bonds and no unshared pairs of electronsE none of the above. Group of answer choices 24 17 16 18 22 - Answered by a verified Tutor.
Electrons in three of them 3 x 23 2.
Lewis Structure Of No3 Nitrate Ion
Lewis Structure For No3 Nitrate Ion
No3 Lewis Structure How To Draw The Lewis Structure For No3 Youtube
Comments
Post a Comment